Unlike a Mixture a Pure Substance Has
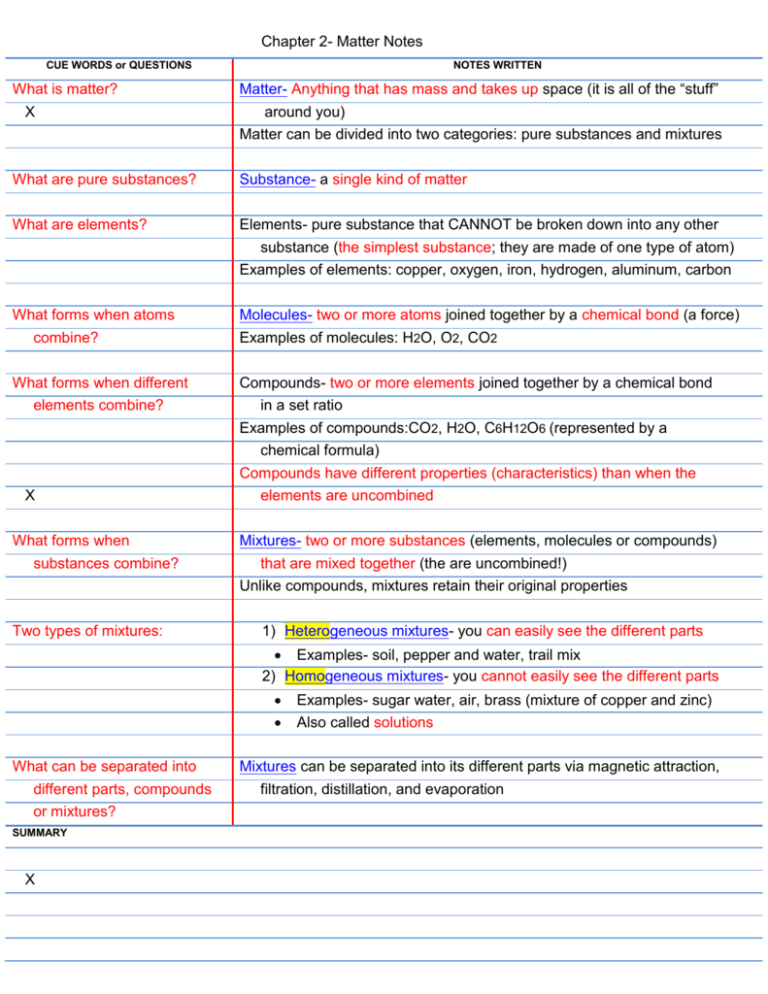
Pure substances are further divided into elements and compounds. The material is no longer a pure substance if it has been mixed with another pure substanceTwo pure substances mixed together are known as a mixturePure hydrogen is a pure substanceSo is pure honey even though it consists of many different types of molecules.

Unlike A Mixture Pure Substance Has Brainly Com
A pure substance consists of only one kind of matter that is all the particles are same.
. Mixtures are physically combined substances that can easily be separated into their primary substances. On the other hand a mixture is a combination of two or more substances in which they retain their properties. An element is a pure.
The matter is divided into two basic categories as pure substance and mixtures. Any mixture can be formed and then separated by physical means into its pure components without changing the identity of those components. Pure substance made up of two or more different elements joined by chemical bonds Made of elements in a specific ratio that is always the same Has a chemical formula H 2 O and CO 2.
Unlike a mixture a pure substance has what. One kind of molecule. A few differences between pure.
The composition of a copper element remains the same no matter what the conditions are. A pure substance has a constant composition. Chemical and physical properties are constant.
It cannot be split into simpler substances by physical means. All physical objects are made up of chemical substances which are unchanging in chemical composition and characteristics. Difference Between Pure Substance and Mixture Composition.
Mixtures can be classified into two types viz. Is flour a pure substance or mixture. In Common Language - Pure means having no adulteration or impurities It means the substance has a single type of particle.
Just like a pure substance- Cu has definite properties like boiling point melting point etc. Mixtures are made up of several substances that are not chemically bonded. Thus the composition is the same throughout.
Molecules can be regarded as the smallest particle of a substance which posses the physical as well as chemical propertie s of that particular substance. Pure Substance vs Mixture. Pure subtance has a different properties while mixture has constant properties.
Pure substances can be elements made up exclusively of one kind of atom or they can be compounds made up of molecules that. Due to these properties copper can not be considered a mixture. A pure sub that cant be broken down into simpler substances is a.
The combination of two or more pure substances is called a mixture. Unlike a mixture a pure substance has. There is a single type of molecule present in a pure substance unlike mixture.
Difference Between Pure Substance and Homogeneous Mixture Pure Substance vs Homogeneous Mixture Matter is composed of different substances like atoms and other molecules that have volume and mass. It has a definite composition and constant properties. Solubility or pure subtance is a solutes that disolves easily than other While mixture or non-uniform you can easily distinguish the components.
All pure substances have characteristic melting and boiling points. A pure substance contain only a single type of molecule. A pure substance is either a compound or an element.
C Pure substance contains two or more molecules while mixture contains. A pure substance is a form of matter that has a definite constant composition and distinctive properties. Unlike a mixture a pure substance has.
A pure substance contain only a single type of molecule. Molecules can be regarded as the combination of one or more atoms. The science of what matter is made of and how it changes is called.
Pure substances can be regarded as substances which. Pure substance that cant be separated into a simpler substance by physical or chemical means Only contains one type of atom Ex. What is an Element.
There can be one or more types of atoms or molecules in the composition of the chemical compound. Pure substances are made of only one matter. The pure substances are further subdivided into elements and compounds.
D Pure substance cannot be separated into any other kind while mixture is a. The major difference between pure substances and mixture is that pure substances have a specific composition of constituent while a mixture is the combination of two or more pure substances. A Mixture contains two or more.
A chemical substance is. Hence Mixture a substance having more than one particle is not called a Pure substance. Pure Substance and Mixture.
A Pure substance has different properties while mixture has constant. It cannot be converted to anything simpler by any means. Substances which have a specific composition and cannot be separated into any constituents are called pure substances.
Heterogeneous and homogeneous mixtures. B Pure substance has combination of substances while mixture has no. The main difference between pure substance and mixture is that the pure substance consists of one kind of component present in the definite composition whereas the mixture is the substance that consists of more than one component which does not exist in the fixed composition.
A mixture is composed of two or more types of matter that can be present in varying amounts. It is a mixture It is a pure substance It is a pure substance.

A Substance That Cannot Be Broken Down Into Simpler Substances Is Ppt Video Online Download
.png?revision=1)
No comments for "Unlike a Mixture a Pure Substance Has"
Post a Comment